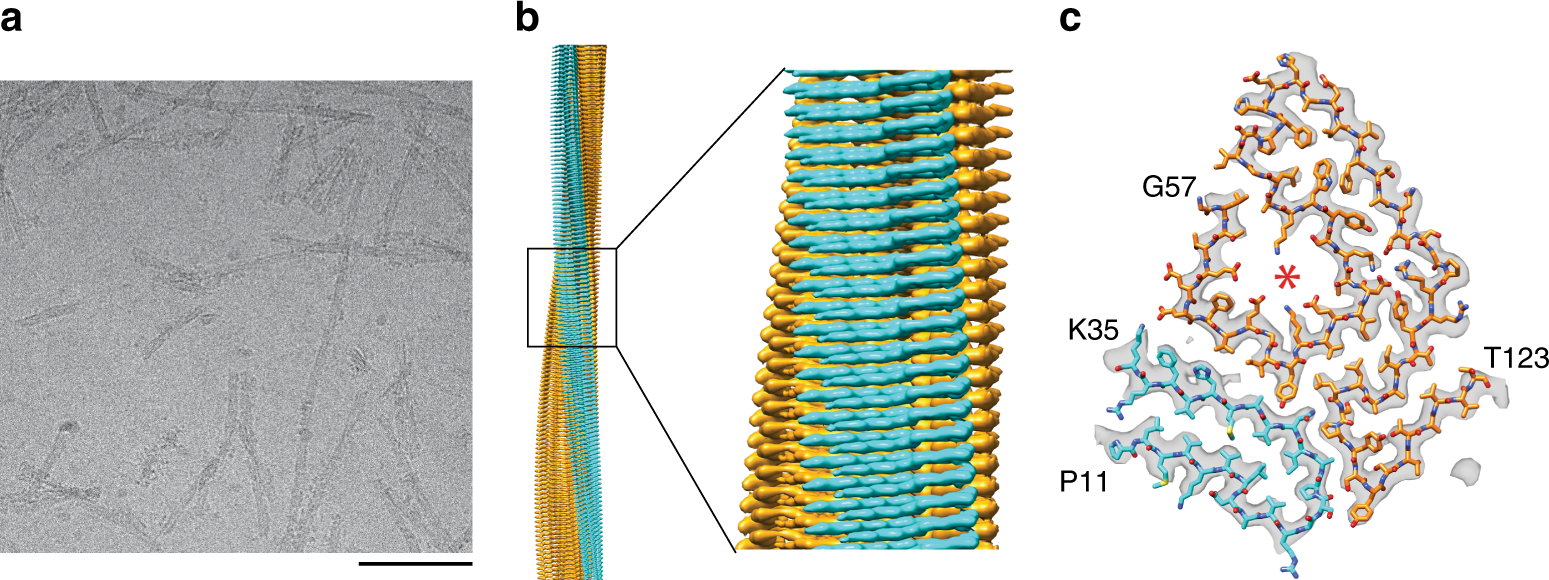
AGGRESCAN: a server for the prediction and evaluation of "hot spots" of aggregation in polypeptides | BMC Bioinformatics | Full Text

Computational prediction of protein aggregation: Advances in proteomics, conformation-specific algorithms and biotechnological applications | Semantic Scholar
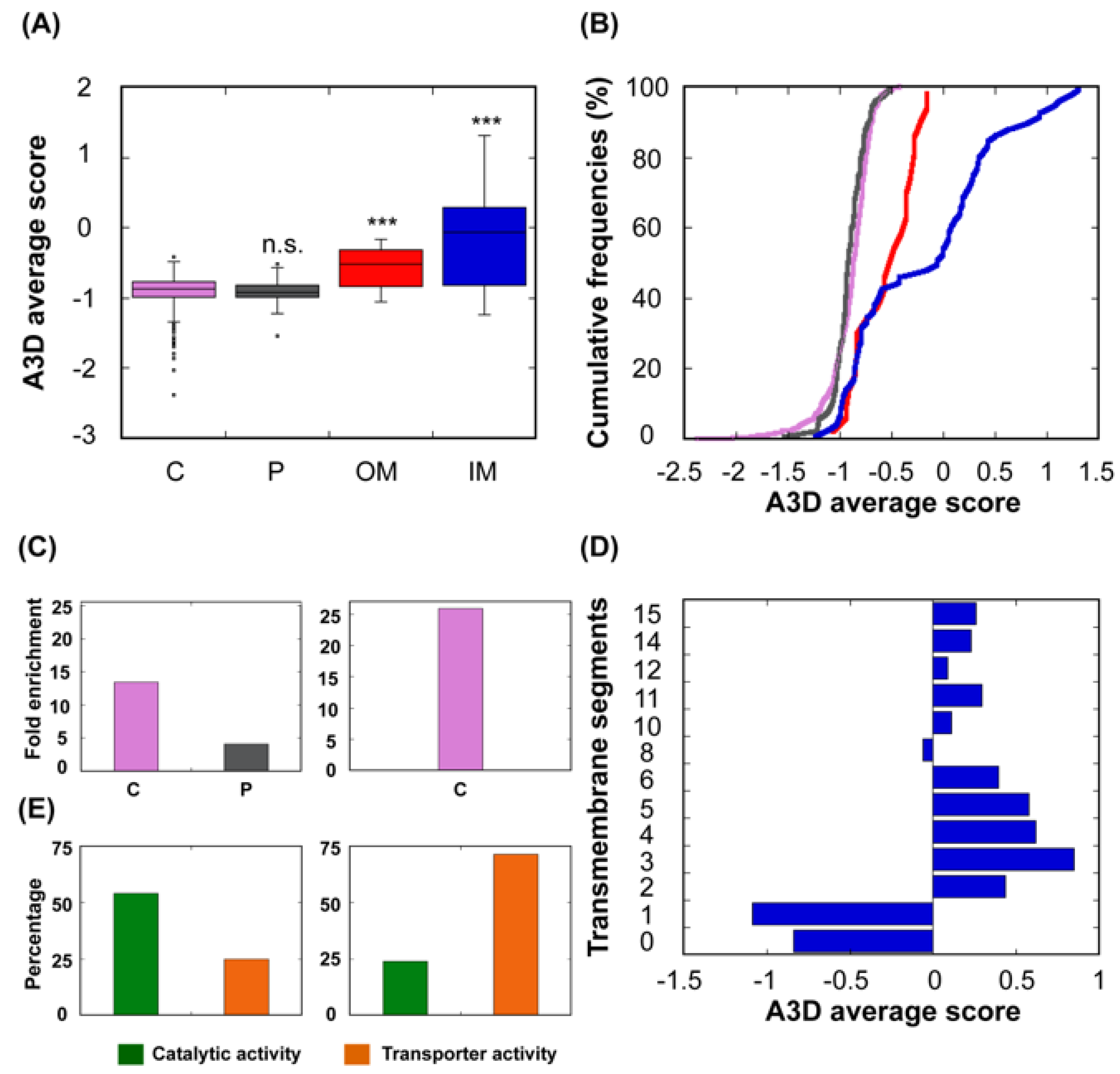
Cells | Free Full-Text | Computational Assessment of Bacterial Protein Structures Indicates a Selection Against Aggregation | HTML
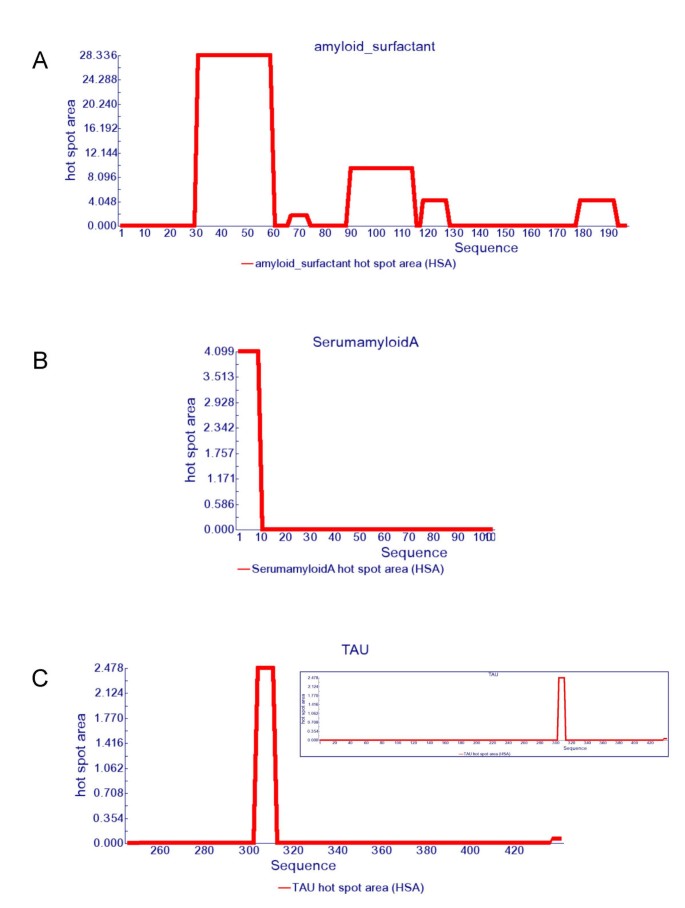
AGGRESCAN: a server for the prediction and evaluation of "hot spots" of aggregation in polypeptides | BMC Bioinformatics | Full Text

Figure 2 from Aggrescan3D (A3D) 2.0: prediction and engineering of protein solubility | Semantic Scholar

Identification of IgG1 Aggregation Initiation Region by Hydrogen Deuterium Mass Spectrometry - Journal of Pharmaceutical Sciences
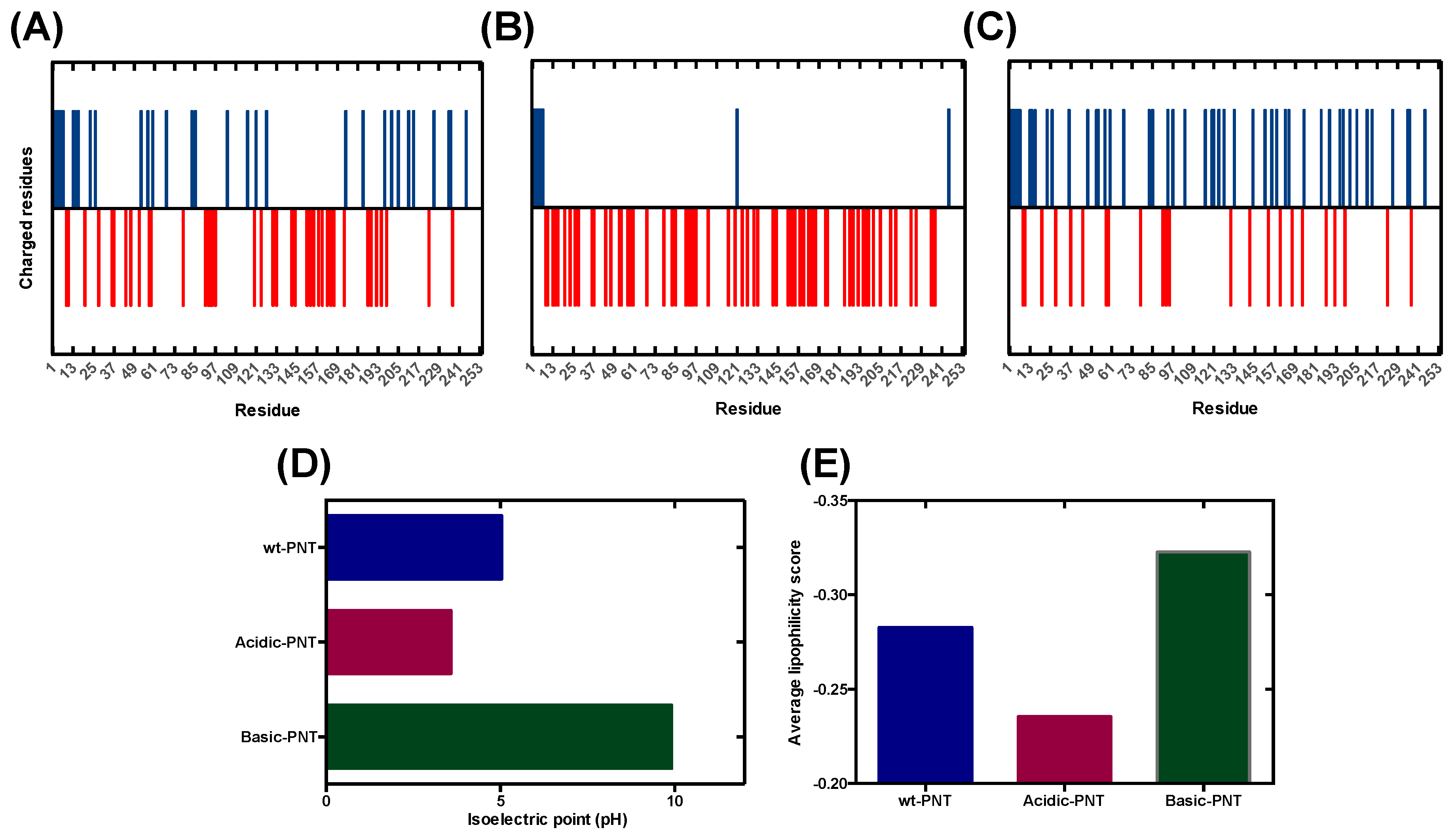
Cells | Free Full-Text | pH-Dependent Aggregation in Intrinsically Disordered Proteins Is Determined by Charge and Lipophilicity | HTML

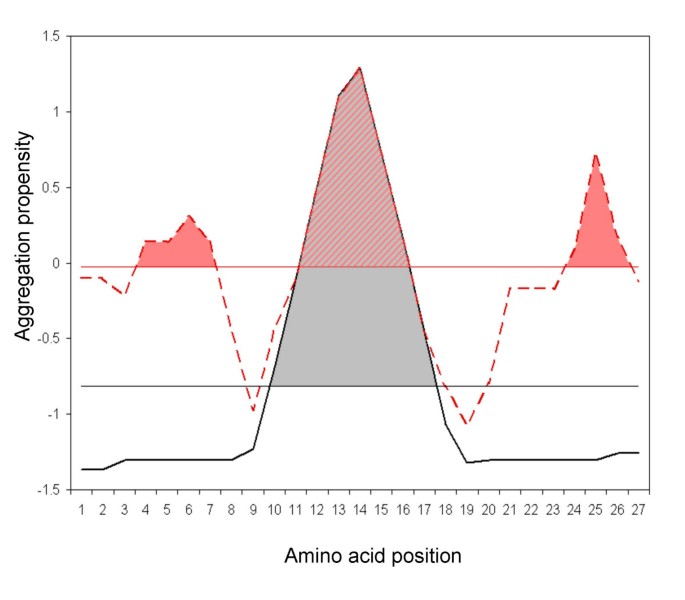
Example of AGGRESCAN output. The red line represents the aggregation... | Download Scientific Diagram
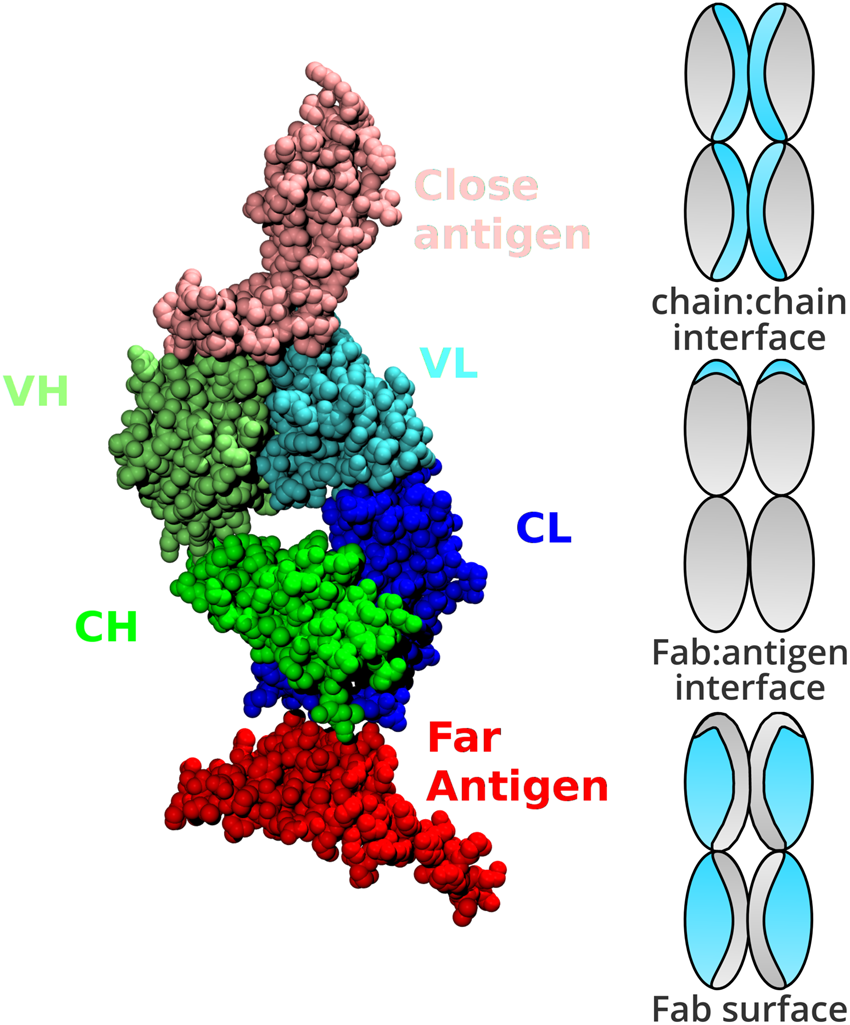
Web-based display of protein surface and pH-dependent properties for assessing the developability of biotherapeutics | Scientific Reports
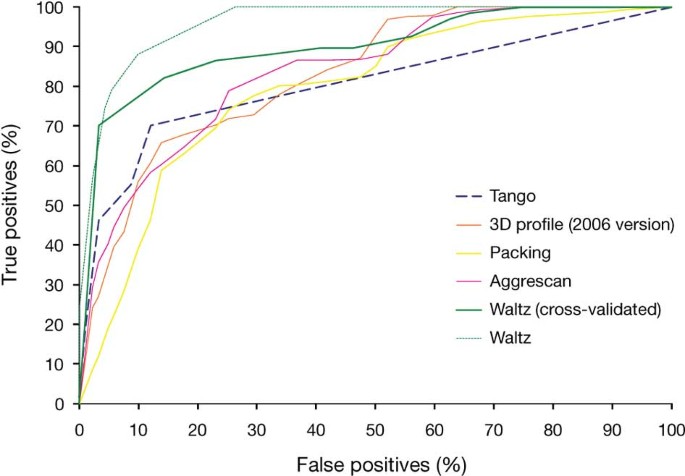
Addendum: Exploring the sequence determinants of amyloid structure using position-specific scoring matrices | Nature Methods

Amyloid properties of the leader peptide of variant B cystatin C: implications for Alzheimer and macular degeneration - Sant'Anna - 2016 - FEBS Letters - Wiley Online Library

Driving Forces for Nonnative Protein Aggregation and Approaches to Predict Aggregation-Prone Regions. | Semantic Scholar

AggScore: Prediction of aggregation‐prone regions in proteins based on the distribution of surface patches - Sankar - 2018 - Proteins: Structure, Function, and Bioinformatics - Wiley Online Library

Using extensional flow to reveal diverse aggregation landscapes for three IgG1 molecules - Willis - 2018 - Biotechnology and Bioengineering - Wiley Online Library

AGGRESCAN: a server for the prediction and evaluation of "hot spots" of aggregation in polypeptides | BMC Bioinformatics | Full Text

Example of AGGRESCAN output. The red line represents the aggregation... | Download Scientific Diagram

Figure 2 from Aggrescan3D (A3D) 2.0: prediction and engineering of protein solubility | Semantic Scholar

AGGRESCAN: a server for the prediction and evaluation of "hot spots" of aggregation in polypeptides | BMC Bioinformatics | Full Text